Products
Contract Manufacturing (API, fluorination, etc.)
Building on an R&D organization with many years of experience in inorganic and organic chemistry and our own fluorination technology, Central Glass has worked with pharmaceutical companies around the world in joint development projects. With the systems in place, we are able to provide contract manufacturing services that support your development and commercialization activities.
-
businesses
-
Search by Product Category
Building on an R&D organization with many years of experience in inorganic and organic chemistry and our own fluorination technology, Central Glass has worked with pharmaceutical companies around the world in joint development projects for active pharmaceutical ingredients. We began globally supplying fluorine-containing drug substance in 1990 and non-fluorine containing drug substance in 2000. We have built a system for GMP-compliant quality assurance and we have the ability to maintain DMF registration.
With the systems in place, we are able to provide contract manufacturing services that support your development and commercialization activities. For example, we can use our facilities where our people are highly skilled in the handling of processes for fluorination or elimination of fluorine. Or Central Glass can work with customers to replace processes that use costly fluorinating agents as in the initial early development phase with economical substitutes developed with a view to commercial production.
Click here to find out about other compounds.
*Contact Central Glass directly for more information.
Production facilities
Central Glass (Ube Plant)
- The production and quality assurance of Central Glass pharmaceutical products is based on GMP. We have set up a GMP system to ensure that we can at all times maintain high levels of production quality control.
- The company undergoes periodic inspections by the US FDA and other regulatory authorities. Our production facilities and quality assurance system have been recognized for their ability to produce high-quality pharmaceutical products.
- Recent FDA inspections:
- September 2014
- June 2018
- September 2023
- Recent local health authority (Yamaguchi prefecture) inspections:
- October2019
- October 2021
- October 2023
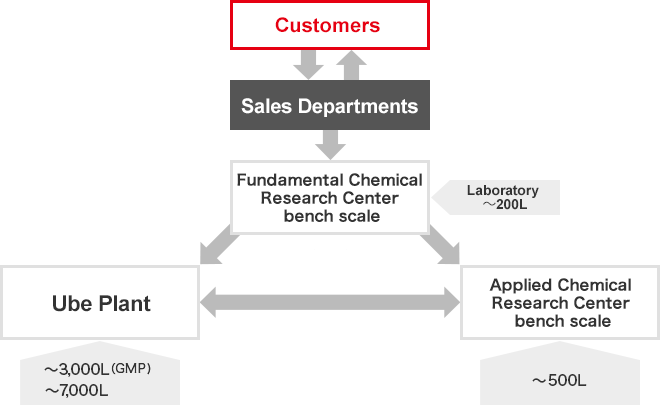
Flow of contract services
- Fluorination technology
- Selective halogen exchange reaction using hydrofluoric acid (HF)
- Electrolytic fluorination
- Deoxyfluorination (-OH→-F)
-
- Direct fluorination using F2 gas
Medi-Chemicals Related Products
Medi-Chemicals Related Research and Development
Contact
Online inquiries
Telephone inquiries
Medi-Chemicals Sales Department
Business hours: 9:00 a.m. - 5:30 p.m.
※Except Saturdays, Sundays, public holidays, August 15, and seasonal holiday periods.
-

Applied Chemicals
We provide basic materials to many industries, such as polyaluminum chloride used as a water treatment agent and rigid urethane blowing agents that do not damage the ozone layer.
-

Medi-Chemicals
The Group has a long history of providing products for pharmaceuticals and medical devices, and we place great emphasis on safety and quality control. We are developing new materials that contribute to improving quality of life.
-
Electronic Materials
We are actively developing materials, with a focus on gases for a wide range of semiconductor processes and photoresist resin materials.
-

Energy Materials
We develop electrolytes and additives for lithium-ion batteries used in electric vehicles and storage batteries. The Central Glass Group provides a stable supply to our customers worldwide from our multiple overseas manufacturing bases.
-

Fertilizers
We manufacture various types of fertilizers, such as rice paddy fertilizers, based on nitrogen, phosphorus, and potassium, which are considered to be the three components of fertilizers.
-

Architectural Glass
We offer products that are healthy, safe, comfortable, and environmentally friendly, such as Eco-Glass, which is effective in improving indoor heating and cooling efficiency, and security glass, to prevent break-ins and theft.
-

Automotive Glass
We are also involved in the eco field to make vehicle interior spaces more comfortable, and we provide high-quality and diverse glass products that contribute to safety, comfort, and design.
-

Glass Fiber
Both continuous glass fiber and glass wool are employed in the automotive, environmental, and other fields.